Targeted Dual Action
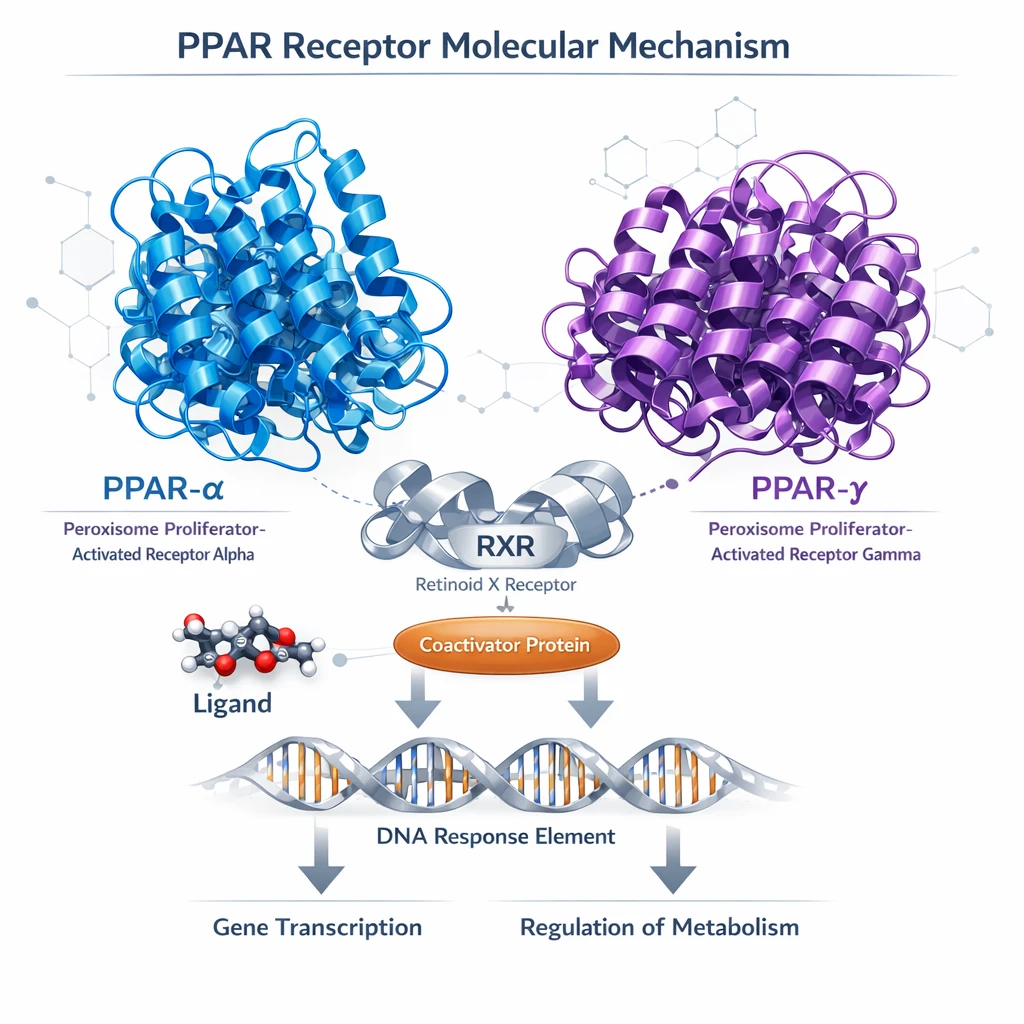
Cardioxil™ binds selectively to PPAR-α receptors in hepatocytes, promoting β-oxidation of fatty acids and reducing plasma triglycerides. Simultaneously, partial PPAR-γ modulation in endothelial cells suppresses NF-κB signaling, lowering vascular inflammation without significant adipogenesis.
PPAR-α Activation
Enhances fatty acid oxidation in hepatocytes, reducing triglyceride levels and improving lipid metabolism
PPAR-γ Modulation
Suppresses vascular inflammation via NF-κB pathway inhibition without promoting adipogenesis
Endothelial Protection
Improves endothelial function and reduces markers of vascular inflammation